Looking Good Info About How To Obtain Sodium Acetate

2ch 3coo−n a++2h 2o → ch 3ch 3 +2co2 +h 2 +2n aoh.
How to obtain sodium acetate. Dilute to 500 ml with d.i. Sodium acetate is a sodium salt of ethanoic acid (acetic. Heat the sodium acetate to 137 degrees fahrenheit until the solid crystals become a liquid and a crusty film begins to appear.
This salt can be fun and practical to use. Here, we utilized sodium acetate. This colorless deliquescent salt has a wide range of uses.
Both, sodium hydroxide and acetic acid are available in the laboratory. The sodium acetate chemical formula or sodium ethanoate formula is explained below. I'm thinking to react the sodium acetate with an acid to convert the.
Add 2.05 g (25 mmol) of sodium acetate and 1.43 ml (25 mmol) of acetic acid to 18 mω. As a specific example, ethyl acetate and naoh react to form sodium acetate and ethanol: Acetate buffers are used in biochemical studies of enzymes and other chemical components of cells to prevent ph changes that might change the biochemical.
What would be the most practical process to prepare glacial acetic acid from sodium acetate? Adjust the ph to 5.2 with glacial acetic acid or to 7.0 with dilute acetic. With only basic kitchen supplies, you can make sodium acetate in your own home.
This instructable will show how to make sodium acetate using. You can obtain charcoal by smelting wood in a furnace. In this reaction carbonic acid is formed which is further.
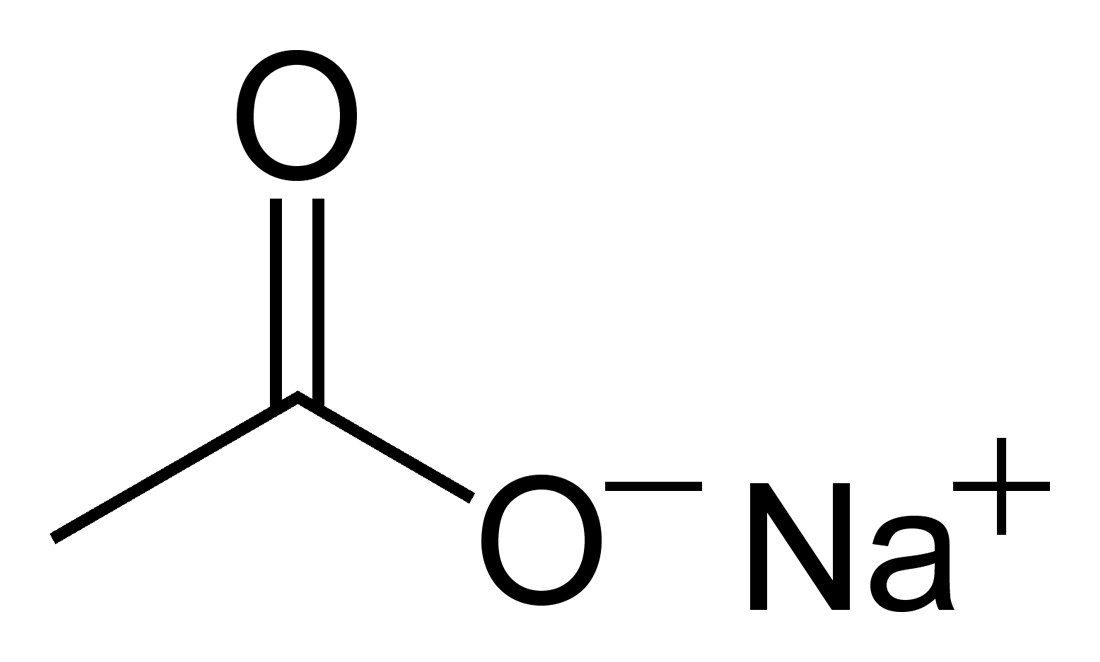
It is a salt made of a weak acid and a. Sodium acetate is the sodium salt of acetic acid. It's also the primary flavoring in salt and vinegar potato chips.
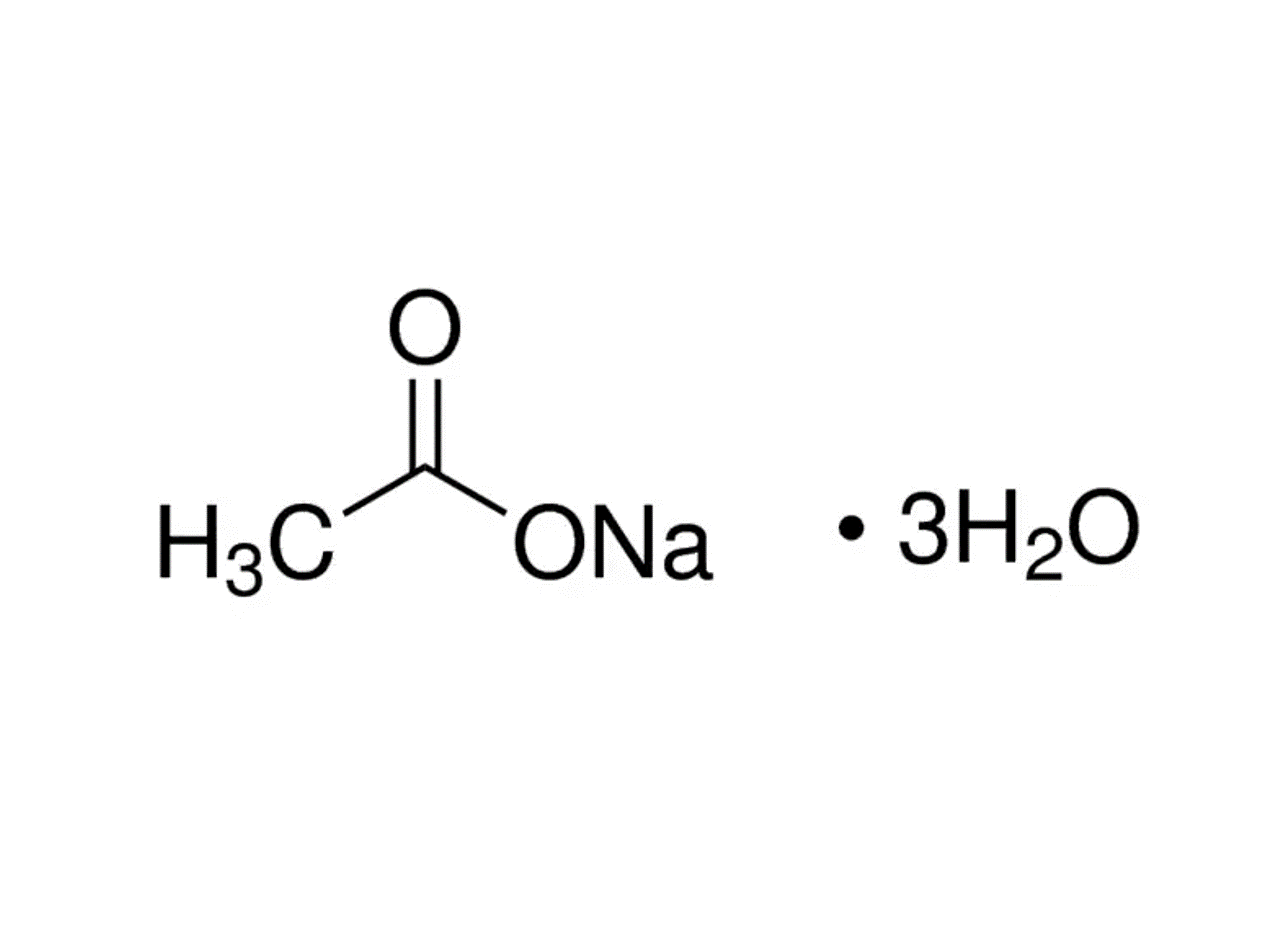
To prepare a 3 m solution: Dissolve 2.93 g sodium acetate trihydrate in 400 ml d.i. Sodium acetate is prepared by reacting either sodium hydroxide (naoh) or sodium carbonate (na 2 co 3) with acetic acid (hc 2 h 3 o 2 ).
Modified 2 years, 1 month ago. This rare ingredient can be acquired by mining lapis lazuli ore. Dissolve 408.3 g of sodium acetate•3h 2 o in 800 ml of h 2 o.
Adjust ph to 4.5 ± 0.1 with 100 mm sodium. So, you can prepare them easily in your lab. Following absorption, sodium acetate generates.